Disordered Protein Movement: Understanding the Fluidic Nature of Proteomics
In recent years, the field of molecular biology has seen a burgeoning interest in disordered proteins and their inherent characteristics. Traditionally, proteins have been characterized by their well-defined three-dimensional structures, which dictate their functional roles in biological processes. However, a significant fraction of cellular proteins exhibit intrinsic disorder, moving beyond traditional paradigms of structural biology. This article aims to elucidate the complexities of disordered protein dynamics, encompassing their biophysical properties, functional implications, and the methodologies employed to study their movement.
The Paradigm Shift: From Structure to Disorder
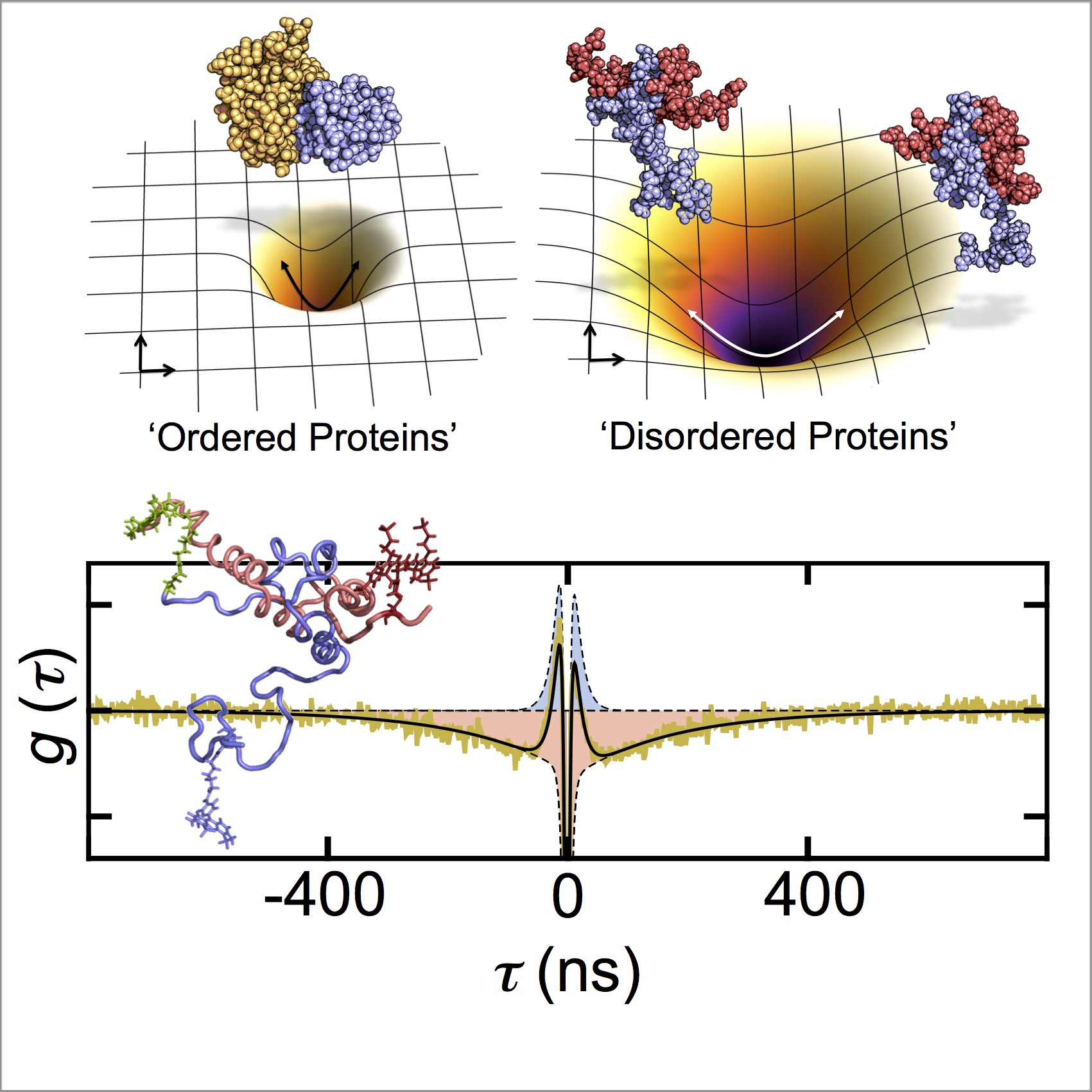
Historically, the study of proteins has largely centered on the “lock-and-key” model, which assumes a rigid, well-defined structure leading to a specific function. Yet, advances in proteomics have significantly reshaped this paradigm. Disordered proteins, often devoid of stable secondary or tertiary structures, present a stark contrast to this conventional perspective, leading to the concept of “structural plasticity.” This property enables disordered proteins to adopt multiple conformations, enhancing their functional versatility across various biological contexts.
The prevalence of disordered proteins is remarkably high, with estimates suggesting that more than 30% of eukaryotic proteins exhibit some degree of disorder. These proteins often participate in critical cellular processes, such as signaling pathways, transcriptional regulation, and molecular assembly. Their dynamic nature allows them to interact with a variety of partner molecules, facilitating an array of biochemical functionalities.
Biophysical Properties of Disordered Proteins
To understand the movement of disordered proteins, one must first delve into their unique biophysical characteristics. Unlike their ordered counterparts, disordered proteins are characterized by rapid conformational fluctuations and a lack of fixed tertiary formation. This dynamic behavior results from an interplay of various physicochemical forces, including hydrophobic interactions, electrostatic forces, and the entropic contributions of chain flexibility.
One notable phenomenon associated with disordered proteins is the concept of “high energy landscapes.” These proteins often exist in a multitude of states separated by low energy barriers, allowing for facile transitions between conformations. The implications of this characteristic are far-reaching. For example, the nucleoporins, which form the nuclear pore complex, rely on disordered regions to facilitate transport processes across the nuclear envelope due to their inherent flexibility.
Furthermore, the biophysical measurement of disordered protein dynamics poses significant challenges. Traditional techniques, such as X-ray crystallography, fall short in addressing the intricate motions that these proteins entail. Instead, advanced methodologies, such as nuclear magnetic resonance spectroscopy (NMR), fluorescence resonance energy transfer (FRET), and single-molecule imaging, have emerged as powerful tools to probe the dynamic landscapes of disordered proteins.
Functional Implications of Disorder
The functional ramifications of disordered proteins extend well beyond mere structural considerations. Their ability to engage in transient interactions enables them to serve as versatile modulators of cellular signaling. For instance, the disordered regions of transcription factors such as p53 or c-Myc allow for the recognition and binding of diverse substrates, assisting in the regulation of gene expression under various cellular conditions.
Moreover, disordered proteins are instrumental in cellular responses to environmental stimuli. The modulation of protein-protein interactions through conformational changes can be pivotal in signaling cascades, where rapid adjustments are often required in response to fluctuating cellular circumstances. This adaptability can be particularly advantageous in stress responses, where disordered proteins might act as “molecular sponges,” absorbing modifications that can trigger downstream signaling events.
Additionally, disordered proteins have been implicated in various diseases, particularly neurodegenerative disorders. Misfolding and aggregation of proteins such as amyloid-beta in Alzheimer’s disease or alpha-synuclein in Parkinson’s disease exemplify the pathological consequences of dysregulated disordered protein dynamics. These insights underscore the critical importance of understanding the movement and interactions of disordered proteins in disease contexts.
Investigating Disordered Protein Dynamics
A comprehensive understanding of disordered protein movement necessitates a multifaceted investigative approach. In recent years, the integration of computational modeling and experimental methodologies has become increasingly prevalent in this field. For example, molecular dynamics simulations can provide valuable insights into the conformational flexibility and potential interaction surfaces of disordered proteins, complementing experimental observations.
Moreover, the advent of innovative imaging techniques, such as cryo-electron tomography and super-resolution microscopy, has revolutionized the study of protein dynamics at the cellular level. These methodologies facilitate the visualization of disordered protein behavior in real-time, offering unparalleled insights into their roles within complex cellular environments.
Understanding the spatial organization of disordered proteins within cellular contexts is equally crucial. The partitioning of these proteins into membrane-less organelles, such as stress granules and nucleoli, underscores their capacity to influence subcellular architecture and functional compartmentalization. Such structures often arise through phase separation, driven by multivalent interactions of disordered proteins, emphasizing the importance of disorder in cellular organization.
Towards a Nuanced Understanding: The Interplay of Disorder and Structure
While the study of disordered proteins represents a remarkable expansion of our understanding of proteomics, it is essential to acknowledge the interplay between order and disorder. Many proteins possess regions of both required stable structure and disordered segments, facilitating a delicate balance that is critical for function. Recent research highlights the notion of “context-dependent disorder,” where the functional relevance of disordered regions is dictated by specific cellular scenarios or interactions.
Thus, a holistic appreciation of disordered protein dynamics encompasses not merely their movement or interaction patterns but also their contextual significance within cellular frameworks. The exploration of this dynamic interplay poses new questions about the fundamental principles underlying proteomics, prompting a reevaluation of classical notions of protein behavior.
Concluding Remarks
The exploration of disordered protein movement offers profound insights into the complexities of molecular biology. The transition from traditional rigid models of protein structure to an understanding of dynamic fluidic behavior elucidates the multifaceted roles that disordered proteins play in cellular processes. As technological advancements continue to pave the way for innovative methodologies, the study of disordered proteins is poised to unveil even deeper understandings of their significance in health and disease.
This nuanced perspective on disordered protein dynamics not only enriches our grasp of molecular biology but also reinforces the interconnectedness of structure and function in the realm of the proteome. Future research endeavors must embrace this complexity, fostering a comprehensive understanding of how these dynamic entities orchestrate biological phenomena with elegance and sophistication.