Understanding the complexity of life often boils down to examining the building blocks that constitute living organisms. One of the most intriguing aspects of molecular biology is the study of proteins, particularly those that are intrinsically disordered. This exploration into the dynamic world of disordered proteins opens avenues for new scientific discoveries and applications in medicine and biotechnology.
Unlike conventional proteins that exhibit a well-defined three-dimensional structure, intrinsically disordered proteins (IDPs) are characterized by their flexibility and lack of a stable conformation. This ambiguity in structure allows IDPs to engage in a myriad of interactions with other biological molecules, thus playing crucial roles in various cellular functions. To attract a younger audience’s interest in the scientific exploration of disordered proteins, it is essential to delve into their unique properties, functional significance, and implications in health and disease.
The following sections will unravel the enigmatic world of IDPs, elucidating their functional versatility and the ramifications of their disordered nature in biological systems.
Unraveling the Mystery: What Are Intrinsically Disordered Proteins?
In stark contrast to the canonical view of proteins as static entities, IDPs defy traditional structural expectations. These proteins exist in a state of conformational diversity, fluctuating between multiple structural states and allowing them to adapt rapidly to different biological contexts. This intrinsic disorder can be attributed to a high proportion of polar and charged amino acids, which stabilize flexible regions and confer pliability.
The significance of IDPs extends beyond their structure; their propensity for disorder is intricately tied to their biological roles. A substantial portion of the human proteome is composed of IDPs, highlighting their evolutionary advantage. Studies estimate that approximately 30-40% of eukaryotic proteins may display intrinsic disorder, indicating a rich landscape of functional diversity.
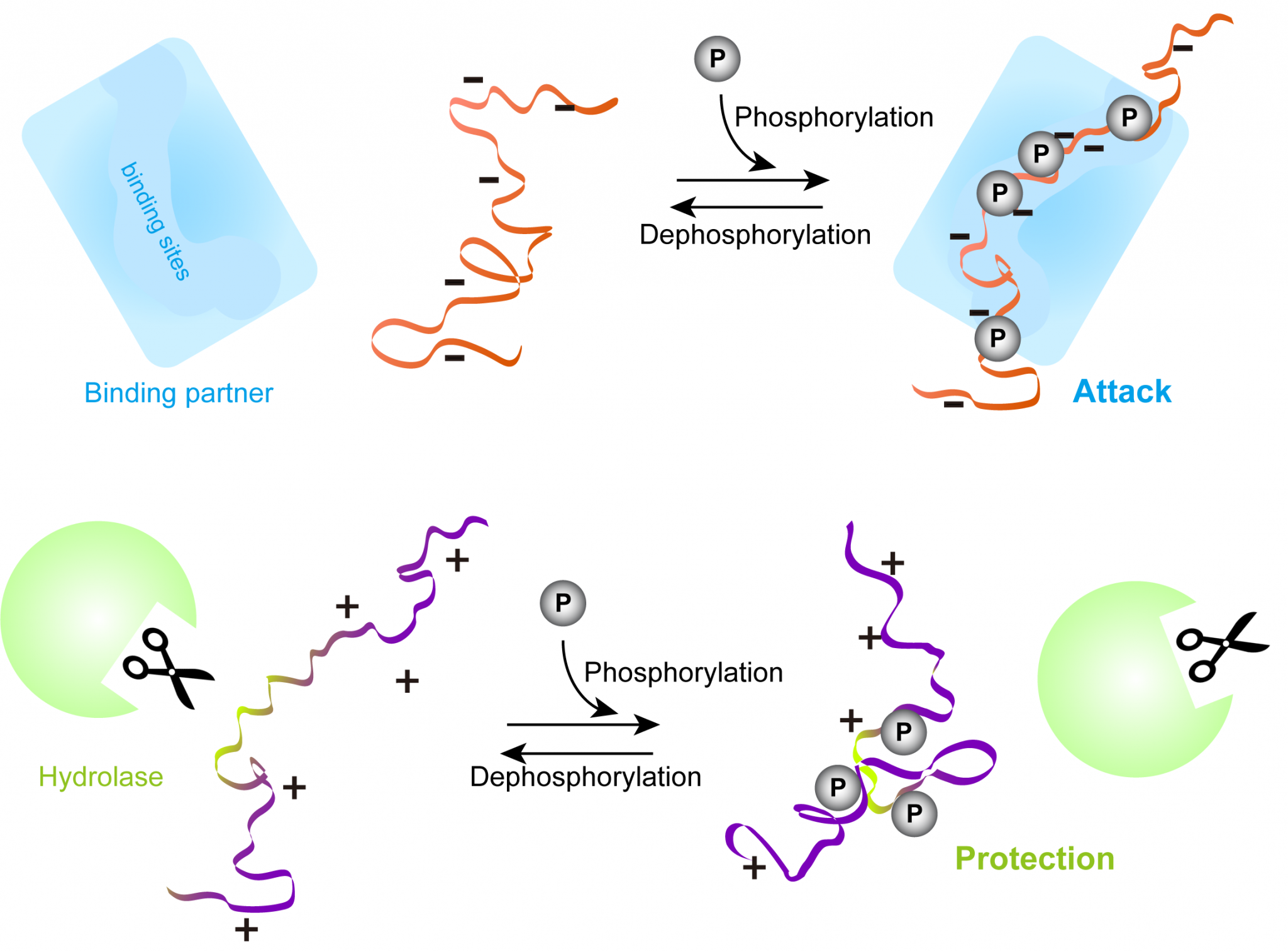
These proteins play pivotal roles in numerous cellular processes, including signal transduction, transcription regulation, and cell cycle control. The elasticity of IDPs serves as a crucial factor in their ability to interact with multiple binding partners and partake in complex signaling networks. This feature enables cells to respond rapidly to environmental stimuli, thus showcasing the importance of these proteins in cellular homeostasis and adaptive responses.
The Versatile Roles of Disordered Proteins in Cellular Functions
One of the alluring aspects of IDPs is their versatility in mediating a variety of molecular interactions. They can engage in transient, yet functionally significant, interactions with other proteins, nucleic acids, or small molecules, thereby modulating numerous biological pathways.
A prime example of this versatility can be observed in signaling proteins. Many IDPs operate as scaffolding components within signaling complexes, facilitating the assembly and disassembly of multiprotein networks. Their flexible nature allows them to bring together the appropriate partners upon signal activation, promoting effective cellular communications. This transient nature of interactions underscores the importance of disordered regions in modulating signal transduction pathways.
Furthermore, IDPs have been reported to possess a role in gene regulation. They can function as transcription factors that bind to specific DNA sequences, altering gene expression in response to developmental cues or stressors. Their ability to undergo phase separation further supports their regulatory functions. Phase separation is a phenomenon where disordered proteins can form membraneless organelles, essentially creating distinct cellular compartments that allow for localized biochemical reactions without the need for membrane-bound structures.
As research continues to unveil the functional tapestry of IDPs, their contributions to the mechanisms of differentiation, signaling, and regulation highlight their paramount importance in maintaining cellular integrity and function.
Health Implications: The Dark Side of Disordered Proteins
While the dynamic and adaptable nature of IDPs offers exciting perspectives, it is essential to acknowledge the potential consequences of their misregulation. The very properties that make them versatile can also lead to pathological scenarios. Many studies have linked IDPs to various diseases, especially neurodegenerative disorders.
In conditions like Alzheimer’s disease, IDPs can misfold and aggregate, leading to the formation of amyloid plaques and neurofibrillary tangles, which contribute to neuronal dysfunction and degeneration. Tangles primarily consist of tau, a protein that serves vital roles in maintaining microtubule stability. When tau becomes hyperphosphorylated, it loses its normal function and adopts an aggregated state, elucidating how dysregulation of IDPs can lead to dire ramifications for neuronal health.
Similarly, in conditions such as Huntington’s and Parkinson’s diseases, the aggregation of disordered proteins disrupts cellular homeostasis and triggers cell death. Understanding the biophysical properties of IDPs in these disorders can foster the development of therapeutic interventions aimed at stabilizing their function or preventing toxic aggregations.
Research is continually unearthing the connections between IDPs and disease, paving the way for novel approaches in diagnostics and drug design. The exploration of small molecules that can modulate the activity of disordered proteins holds promise in targeting pathways affected in various disorders, thereby offering hope for innovative treatments.
Harnessing the Power of Disordered Proteins: Future Perspectives
The increasing recognition of IDPs and their roles in cellular functions presents enormous potential in the realms of biotechnology and medicine. Engineering IDPs to create synthetic biological systems could lead to advancements in diagnostics, therapeutics, and even materials science.
Future research may also focus on elucidating the precise mechanisms by which IDPs engage in their myriad interactions. Advances in techniques such as nuclear magnetic resonance (NMR) spectroscopy, cryo-electron microscopy, and advanced computational modeling are invaluable for studying these complex proteins. By characterizing the structure-function relationships of IDPs, scientists can unravel the fine-tuning required for their regulatory activities.
Furthermore, the knowledge gained from studying IDPs in basic research can ultimately translate into translational approaches, with the possibility of developing pharmacological agents that target IDPs implicated in disease. This innovative line of inquiry reflects a burgeoning area of study ripe for exploration.
In Conclusion
The study of intrinsically disordered proteins is a captivating and continuously evolving field that invites curiosity and exploration. By understanding the unique characteristics and functional roles of IDPs, we can appreciate the complexity of biological systems, paving the way for groundbreaking advancements in health and medicine. Engaging the younger audience in this scientific pursuit not only highlights the beauty of molecular biology but also imparts a sense of responsibility for nurturing the future of scientific inquiry. As the interdependence of structure and function unfolds, the story of disordered proteins continues to captivate and inspire the next generation of scientists.